| Structure | Name/CAS No. | Articles |
|---|---|---|
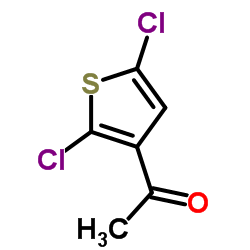 |
3-Acetyl-2,5-dichlorothiophene
CAS:36157-40-1 |
|
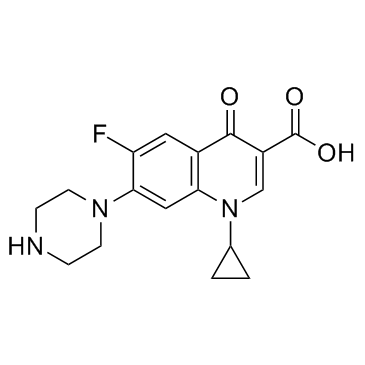 |
Ciprofloxacin
CAS:85721-33-1 |
|
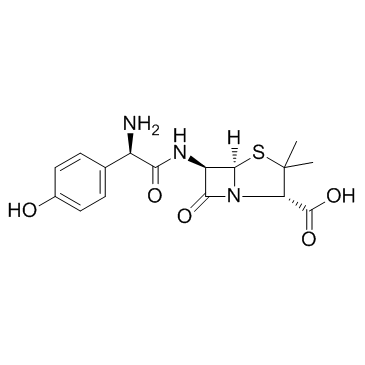 |
Amoxicillin
CAS:26787-78-0 |