Differences in the allosteric modulation by phenytoin of the binding properties of the sigma1 ligands [3H](+)-pentazocine and [3H]NE-100.
Enrique J Cobos, Gema Lucena, José M Baeyens, Esperanza Del Pozo
Index: Synapse 59(3) , 152-61, (2006)
Full Text: HTML
Abstract
The present study evaluated the effects of phenytoin (DPH) on the binding to synaptosomal fraction membranes from guinea pig brain of the prototypic sigma1 (sigma1) receptor agonist [3H](+)-pentazocine and the putative sigma1 antagonist [3H]NE-100. Equilibrium and binding kinetics studies were done. The order of affinity of 12 sigma1 ligands for binding sites labeled with [3H](+)-pentazocine correlated well with their order of affinity for sites labeled with [3H]NE-100, suggesting that both radioligands label the same receptor. Phenytoin increased the binding of [3H](+)-pentazocine, enhancing its affinity (K(D) value) for sigma1 receptors and decreasing its dissociation rate from these receptors. The maximal number of receptors (B(max) value) labeled with [3H](+)-pentazocine was not changed. In contrast, phenytoin decreased the specific binding and maximal number of receptors labeled with [3H]NE-100, and increased its dissociation rate from sigma1 receptors. The affinity of this radioligand for sigma1 receptors was not modified. In conclusion, phenytoin behaved as a positive allosteric modulator on the binding of [3H](+)-pentazocine, whereas it negatively modulated the binding of [3H]NE-100. These results add evidence in favor of the use of phenytoin in vitro to distinguish between agonists and antagonists of sigma1 receptors.(c) 2005 Wiley-Liss, Inc.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
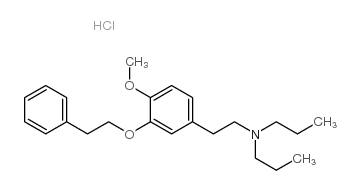 |
NE 100 hydrochloride
CAS:149409-57-4 |
C23H34ClNO2 |
|
Diverse regulation of IP3 and ryanodine receptors by pentazo...
2013-10-15 [Am. J. Physiol. Heart Circ. Physiol. 305(8) , H1201-12, (2013)] |
|
A sigma-1 receptor antagonist (NE-100) prevents tunicamycin-...
2013-05-17 [Biochem. Biophys. Res. Commun. 434(4) , 904-9, (2013)] |
|
Prediction of drug-drug interactions for AUCoral of high cle...
2006-02-01 [Curr. Drug Metab. 7(2) , 135-46, (2006)] |
|
Chloroquine inhibits glutamate-induced death of a neuronal c...
2011-11-01 [J. Neurochem. 119(4) , 839-47, (2011)] |
|
A novel adamantane derivative attenuates retinal ischemia-re...
2006-04-24 [Eur. J. Pharmacol. 536(1-2) , 200-3, (2006)] |