| Structure | Name/CAS No. | Articles |
|---|---|---|
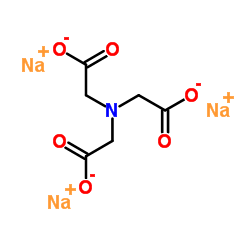 |
SODIUM NITRILOTRIACETATE
CAS:5064-31-3 |
|
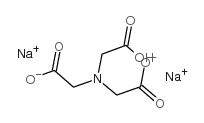 |
Disodium nitrilotriacetate
CAS:15467-20-6 |
|
 |
Xanthine amine congener
CAS:96865-92-8 |
|
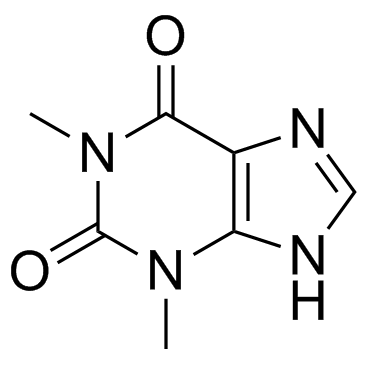 |
Theophylline
CAS:58-55-9 |
|
 |
DPCPX
CAS:102146-07-6 |
|
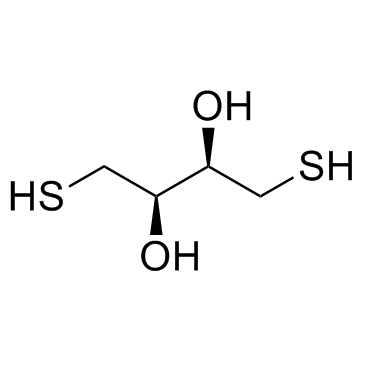 |
DL-Dithiothreitol
CAS:3483-12-3 |
|
 |
Ethylenediaminetetraacetic acid
CAS:60-00-4 |