D1-like dopamine receptor activation and natriuresis by nitrocatechol COMT inhibitors.
M A Vieira-Coelho, P Gomes, M P Serrão, P Soares-da-Silva
Index: Kidney Int. 59(5) , 1683-94, (2001)
Full Text: HTML
Abstract
In recent years, several nitrocatechol derivatives (tolcapone, entacapone, and nitecapone) have been developed and found to be highly selective and potent inhibitors of catechol-O-methyltransferase (COMT). More recently, natriuretic properties were described for two of these compounds (entacapone and nitecapone), although this was not accompanied by enhanced urinary excretion of dopamine. We hypothesized that nitrocatechol derivatives stimulate D1-like dopamine receptors.Adult male Wistar rats were treated with a nitrocatechol COMT inhibitor (entacapone, tolcapone, or nitecapone, 30 mg/kg, orally), and the urinary excretion of dopamine and sodium was quantitated. The interaction of nitrocatechol derivatives with D1-like receptors was evaluated by their ability to displace [3H]-Sch23390 binding from membranes of rat renal cortex and cAMP production in opossum kidney (OK) cells.Urinary excretion of sodium (micromol/h) was markedly increased by all three nitrocatechol derivatives: vehicle, 55.0 +/- 5.6; entacapone, 98.4 +/- 9.3; tolcapone, 97.5 +/- 9.3; and nitecapone, 120.5 +/- 12.6. Pretreatment with the selective D1 antagonist Sch 23390 (60 microg/kg) completely prevented their natriuretic effects. Nitecapone and tolcapone were equipotent (IC50s of 48 and 42 micromol/L) and more potent than entacapone and dopamine (IC50s of 107 and 279 micromol/L) in displacing [3H]-Sch23390 binding. In OK cells, all three nitrocatechol derivatives significantly increased cAMP accumulation and reduced Na(+)/H(+) exchange and Na(+),K(+)-ATPase activities, this being prevented by a blockade of D1-like receptors.Stimulation of D1-like dopamine receptors and inhibition of Na(+)/H(+) exchange and Na(+),K(+)-ATPase activities by nitrocatechol COMT inhibitors may contribute to natriuresis produced by these compounds.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
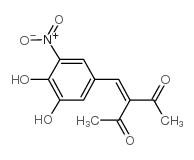 |
Nitecapone
CAS:116313-94-1 |
C12H11NO6 |
|
Extending levodopa action: COMT inhibition.
1998-06-01 [Neurology 50(6 Suppl 6) , S27-32; discussion S44-8, (1998)] |
|
The Helicobacter felis mouse model in assessing anti-Helicob...
1996-04-01 [Scand. J. Gastroenterol. 31(4) , 334-8, (1996)] |
|
Nitecapone and selegiline as effective adjuncts to L-DOPA in...
2002-01-01 [Methods Find. Exp. Clin. Pharmacol. 24(1) , 23-9, (2002)] |
|
Dopamine is metabolised by different enzymes along the rat n...
2005-06-01 [Pflugers Arch. 450(3) , 185-91, (2005)] |
|
The effect of nitecapone on early graft function in experime...
2000-08-01 [Scand. Cardiovasc. J. 34(4) , 415-20, (2000)] |