O-Alkylation of cupferron: aiming at the design and synthesis of controlled nitric oxide releasing agents.
Y Hou, W Xie, A J Janczuk, P G Wang
Index: J. Org. Chem. 65(14) , 4333-7, (2000)
Full Text: HTML
Abstract
O-Alkylation of N-nitroso-N-phenylhydroxylamine ammonium salt (cupferron) was studied for the synthesis of novel nitric oxide (NO) releasing agents. The alkylation occurred regioselectively at the terminal oxygen, leading to a single product N-(alkyloxy)-N'-phenyldiimide N'-oxide as indicated by NMR and X-ray analysis. The O-alkyl derivatives exhibited significantly improved stability compared to their parent compound, cupferron. It was demonstrated that the cupferron O-alkyl derivatives could function as photoreleasing NO donor compounds. N-(N"-acetylphenylalanylmethylenyloxy)-N'-phenyldiimide N'-oxide), which linked the cupferron portion with an amino acid via an acetal moiety, was synthesized as an model NO prodrug where controlled NO release would occur either by increasing pH or by a protease-catalyzed hydrolysis.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
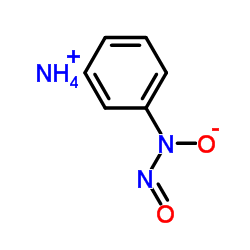 |
Cupferron
CAS:135-20-6 |
C6H9N3O2 |
|
Intermediate analogue inhibitors of mandelate racemase: N-Hy...
2007-01-01 [Bioorg. Med. Chem. Lett. 17 , 105-8, (2007)] |
|
Celecoxib prodrugs possessing a diazen-1-ium-1,2-diolate nit...
2010-08-01 [Bioorg. Med. Chem. Lett. 20 , 4544-9, (2010)] |
|
Synthesis and characterization of lithium oxonitrate (LiNO).
2013-01-01 [J. Inorg. Biochem. 118 , 128-33, (2013)] |
|
Generation of nitric oxide by enzymatic oxidation of N-hydro...
1985-04-10 [J. Biol. Chem. 260(7) , 4069-74, (1985)] |
|
A "dual-function" photocage releasing nitric oxide and an an...
2009-07-13 [Chemistry 15(28) , 6802-6, (2009)] |