High-performance liquid chromatography/tandem mass spectrometry method for the determination of arbidol in human plasma.
Xin Xiong, Suodi Zhai
Index: J. AOAC Int. 94(4) , 1100-5, (2011)
Full Text: HTML
Abstract
An HPLC/MS/MS method for the determination of arbidol in human plasma was developed. Arbidol and internal standard (loratadine) were extracted from alkaline plasma with tert-butyl methyl ether and analyzed on a Zorbax SB C18 column (30 x 2.1 mm id, 3.5 microm particle size). The detection was by monitoring arbidol at m/z 479.1 --> 434.1 and the internal standard at m/z 383.2 --> 337.2. The method was validated according to U.S. Food and Drug Administration guidelines. The calibration curve was linear over the range of 0.5-500 ng/mL using a 100 microL sample volume. The intraday and interday precisions were less than 6.5%, and acceptable values were obtained for accuracy, recovery, and sensitivity. The developed method was selective, simple, sensitive, and easily applicable.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
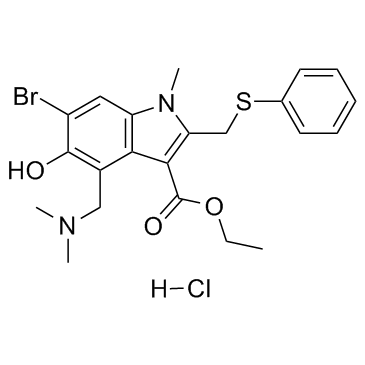 |
Arbidol HCl
CAS:131707-23-8 |
C22H26BrClN2O3S |
|
[Potentiation of NO-dependent activation of soluble guanylyl...
2013-01-01 [Biomed. Khim. 59(3) , 295-304, (2013)] |
|
Arbidol exhibits strong inhibition towards UDP-glucuronosylt...
2013-12-01 [Pharmazie 68(12) , 945-50, (2013)] |
|
Pharmacokinetics, metabolism, and excretion of the antiviral...
2013-04-01 [Antimicrob. Agents Chemother. 57(4) , 1743-55, (2013)] |
|
Glucuronidation of the broad-spectrum antiviral drug arbidol...
2013-04-01 [J. Pharm. Pharmacol. 65(4) , 521-7, (2013)] |
|
Membranotropic effects of arbidol, a broad anti-viral molecu...
2010-07-01 [J. Phys. Chem. B 114(25) , 8544-54, (2010)] |