Pharmacokinetics, metabolism, and excretion of the antiviral drug arbidol in humans.
Pan Deng, Dafang Zhong, Kate Yu, Yifan Zhang, Ting Wang, Xiaoyan Chen
Index: Antimicrob. Agents Chemother. 57(4) , 1743-55, (2013)
Full Text: HTML
Abstract
Arbidol is a broad-spectrum antiviral drug that is used clinically to treat influenza. In this study, the pharmacokinetics, metabolism, and excretion of arbidol were investigated in healthy male Chinese volunteers after a single oral administration of 200 mg of arbidol hydrochloride. A total of 33 arbidol metabolites were identified in human plasma, urine, and feces. The principal biotransformation pathways included sulfoxidation, dimethylamine N-demethylation, glucuronidation, and sulfate conjugation. The major drug-related component in the plasma was sulfinylarbidol (M6-1), followed by unmetabolized arbidol, N-demethylsulfinylarbidol (M5), and sulfonylarbidol (M8). The exposures of M5, M6-1, and M8, as determined by the metabolite-to-parent area under the plasma concentration-time curve from 0 to t (AUC(0-t)) ratio, were 0.9 ± 0.3, 11.5 ± 3.6, and 0.5 ± 0.2, respectively. In human urine, glucuronide and sulfate conjugates were detected as the major metabolites, accounting for 6.3% of the dose excreted within 0 to 96 h after drug administration. The fecal specimens mainly contained the unchanged arbidol, accounting for 32.4% of the dose. Microsomal incubation experiments demonstrated that the liver and intestines were the major organs that metabolize arbidol in humans. CYP3A4 was the major isoform involved in arbidol metabolism, whereas the other P450s and flavin-containing monooxygenases (FMOs) played minor roles. These results indicated possible drug interactions between arbidol and CYP3A4 inhibitors and inducers. Further investigations are needed to understand the importance of M6-1 in the efficacy and safety of arbidol, because of its high plasma exposure and long elimination half-life (25.0 h).
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
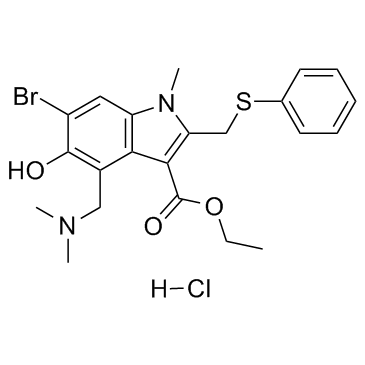 |
Arbidol HCl
CAS:131707-23-8 |
C22H26BrClN2O3S |
|
[Potentiation of NO-dependent activation of soluble guanylyl...
2013-01-01 [Biomed. Khim. 59(3) , 295-304, (2013)] |
|
Arbidol exhibits strong inhibition towards UDP-glucuronosylt...
2013-12-01 [Pharmazie 68(12) , 945-50, (2013)] |
|
Glucuronidation of the broad-spectrum antiviral drug arbidol...
2013-04-01 [J. Pharm. Pharmacol. 65(4) , 521-7, (2013)] |
|
Membranotropic effects of arbidol, a broad anti-viral molecu...
2010-07-01 [J. Phys. Chem. B 114(25) , 8544-54, (2010)] |
|
Synthesis and anti-hepatitis C virus activity of novel ethyl...
2010-08-15 [Bioorg. Med. Chem. 18(16) , 6143-8, (2010)] |