Synthesis and anti-hepatitis C virus activity of novel ethyl 1H-indole-3-carboxylates in vitro.
Grazia Sellitto, Aurora Faruolo, Paolo de Caprariis, Sergio Altamura, Giacomo Paonessa, Gennaro Ciliberto
Index: Bioorg. Med. Chem. 18(16) , 6143-8, (2010)
Full Text: HTML
Abstract
A series of ethyl 1H-indole-3-carboxylates 9a(1)(-)(6) and 9b(1)(-)(2) were prepared and evaluated in Huh-7.5 cells. Most of the compounds exhibited anti-hepatitis C virus (HCV) activities at low concentration. The selectivity indices of inhibition on entry and replication of compounds 9a(2) (>10; >16.7) and 9b(1) (>6.25; >16.7) were higher than those of the other evaluated compounds, including the lead compound Arbidol (ARB, 6; 15). Moreover, the selective index of inhibition on entry of compound 9a(3) (>6.25) was higher than that of ARB (6). Of these three initial hits, compound 9a(2) was the most potent.Copyright 2010 Elsevier Ltd. All rights reserved.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
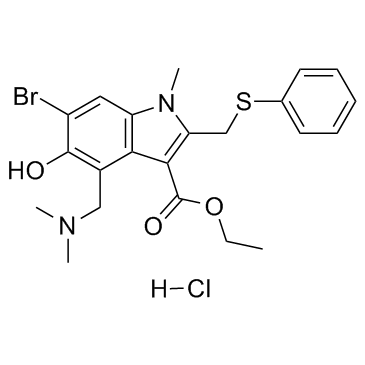 |
Arbidol HCl
CAS:131707-23-8 |
C22H26BrClN2O3S |
|
[Potentiation of NO-dependent activation of soluble guanylyl...
2013-01-01 [Biomed. Khim. 59(3) , 295-304, (2013)] |
|
Arbidol exhibits strong inhibition towards UDP-glucuronosylt...
2013-12-01 [Pharmazie 68(12) , 945-50, (2013)] |
|
Pharmacokinetics, metabolism, and excretion of the antiviral...
2013-04-01 [Antimicrob. Agents Chemother. 57(4) , 1743-55, (2013)] |
|
Glucuronidation of the broad-spectrum antiviral drug arbidol...
2013-04-01 [J. Pharm. Pharmacol. 65(4) , 521-7, (2013)] |
|
Membranotropic effects of arbidol, a broad anti-viral molecu...
2010-07-01 [J. Phys. Chem. B 114(25) , 8544-54, (2010)] |