| Structure | Name/CAS No. | Articles |
|---|---|---|
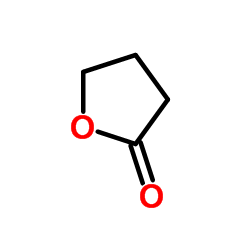 |
gamma-Butyrolactone
CAS:96-48-0 |
|
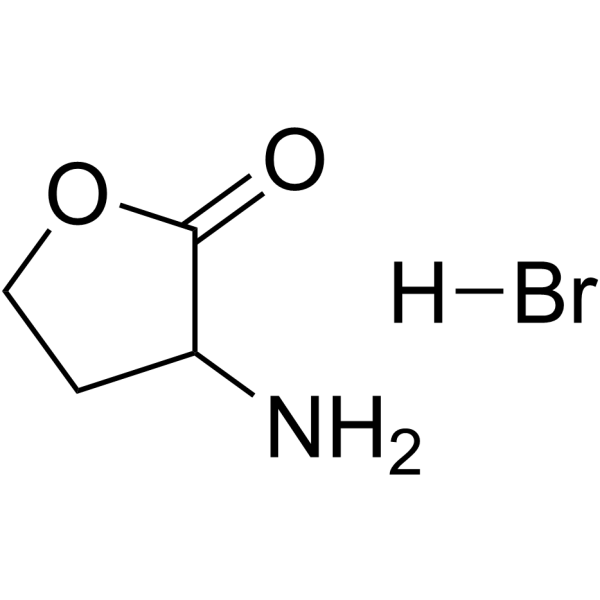 |
2(3H)-Furanone,3-aminodihydro-, hydrobromide (1:1)
CAS:6305-38-0 |
|
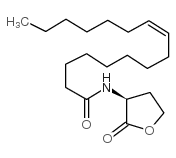 |
2(3H)-Furanone,3-aminodihydro-(8CI,9CI)
CAS:1192-20-7 |
|
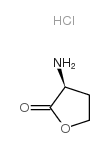 |
L-Homoserine lactone hydrochloride
CAS:2185-02-6 |
|
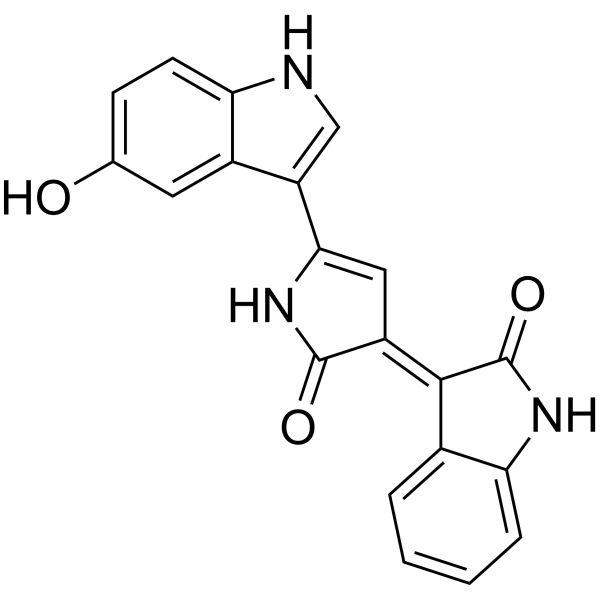 |
Violacein
CAS:548-54-9 |
|
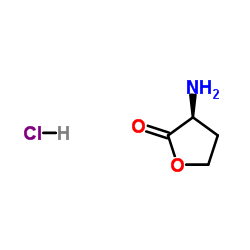 |
L-homoserine lactone hydrochloride
CAS:2185-03-7 |