| Structure | Name/CAS No. | Articles |
|---|---|---|
 |
Hydrochloric acid
CAS:7647-01-0 |
|
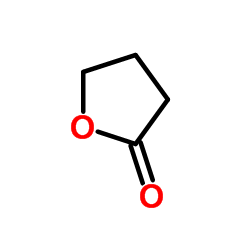 |
gamma-Butyrolactone
CAS:96-48-0 |
|
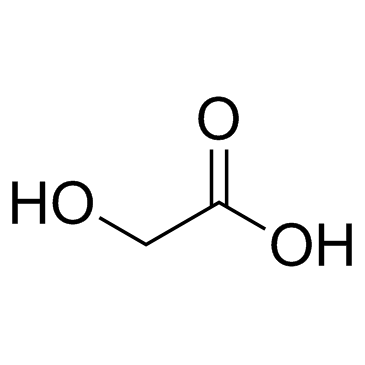 |
Glycolic acid
CAS:79-14-1 |