| Structure | Name/CAS No. | Articles |
|---|---|---|
 |
H-Phe-Gly-OH
CAS:721-90-4 |
|
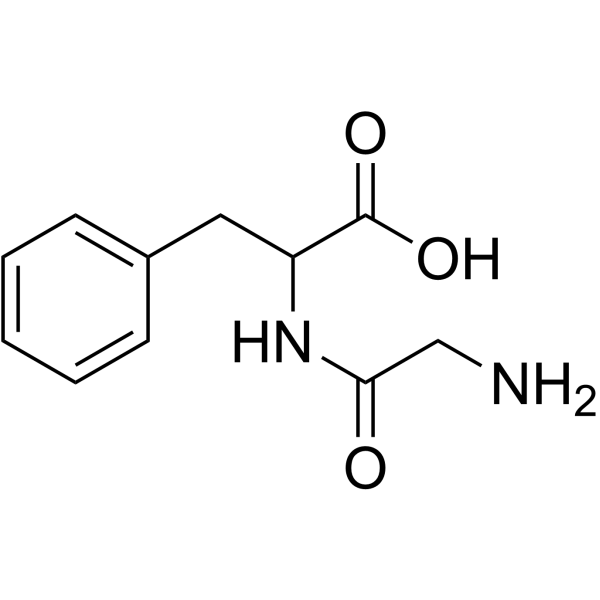 |
H-Gly-DL-Phe-OH
CAS:721-66-4 |
|
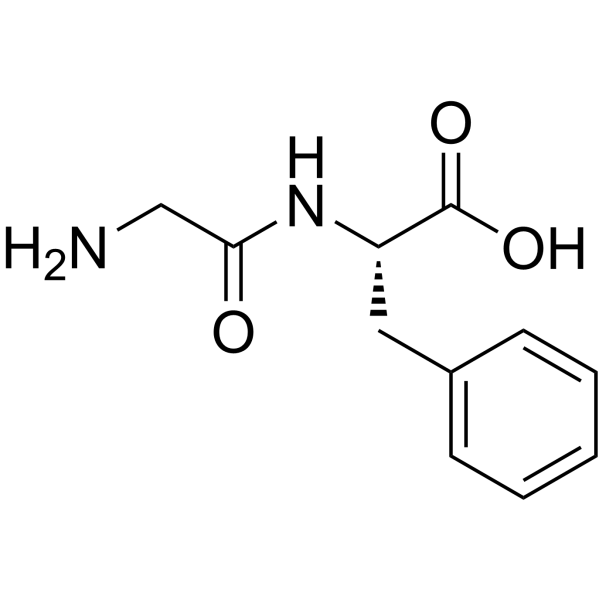 |
H-Gly-Phe-OH
CAS:3321-03-7 |