Convulsant effects of some xanthine derivatives in genetically epilepsy-prone rats.
A De Sarro, S Grasso, M Zappala, F Nava, G De Sarro
Index: Naunyn Schmiedebergs Arch. Pharmacol. 356(1) , 48-55, (1997)
Full Text: HTML
Abstract
The behavioural and electrocorticographic (ECoG) convulsant effects of several xanthine derivatives injected intraperitoneally (i.p.) were studied in genetically-epilepsy prone rats. The aim of the study was to evaluate the relationship among convulsant potency, molecular structure and lipophilicity of some xanthines. Animals were injected i.p. with various doses (250-1000 micromol/kg) and a different convulsant potency was observed among the various xanthines tested. IBMX (3-isobutyl-1-methylxanthine), theophylline (1,3-dimethylxanthine) and caffeine (1,3,7-trimethylxanthine) induced an epileptogenic pattern that consisted in an initial phase characterized by wet-dog shakes followed by head tremor, nodding, clonic convulsion and they appeared to be the most potent xanthines among those studied. During seizures, the electrocortical activity was usually characterized by single or multiple sharp- or spike-wave episodes followed by polyspike discharges. After the highest doses of IBMX, theophylline and caffeine, the animals react with falling down, transient tonic clonic seizures, escape response and generalized seizures followed by post-ictal period. Equimolar doses of 8-chlorotheophylline and theobromine (3,7-dimethylxanthine) produced less evident epileptic responses in comparison to previous compounds, whereas no epileptic signs were observed following the administration of enprofylline (3-propylxanthine), etofylline [7-(2-hydroxyethyl)theophylline], diprophylline [7-(2,3-dihydroxy-propyl)theophylline] and doxofylline [7-(1,3-dioxolan-2-ylmethyl) theophylline]. Lipophylicity of the compounds was determined, but no convincing correlations were found between the rank order of lipophilicities and the convulsant potencies of the compounds studied. On the other hand, structure-activity relationship was also investigated. We suggest that the substitution pattern on the xanthine nucleus may explain, in part, the different convulsant potency of the compounds studied. Furthermore, a selective antagonism of adenosine subtype receptors should be considered.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
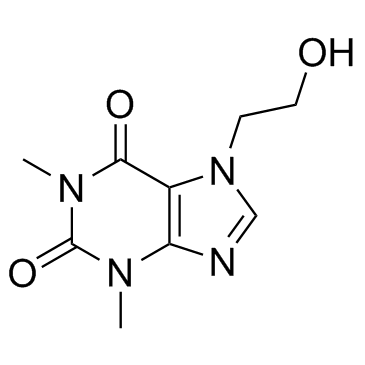 |
7-(2-Hydroxyethyl)theophylline
CAS:519-37-9 |
C9H12N4O3 |
|
Insights into binding modes of adenosine A(2B) antagonists w...
2010-08-01 [Eur. J. Med. Chem. 45 , 3459-71, (2010)] |
|
A simple and rapid HPLC/UV method for the simultaneous quant...
2007-04-01 [J. Chromatogr. B. Analyt. Technol. Biomed. Life Sci. 848(2) , 271-6, (2007)] |
|
Pharmacological studies in animals of beta-hydroxyethyltheop...
1991-05-01 [Methods Find. Exp. Clin. Pharmacol. 13(4) , 289-99, (1991)] |
|
Investigation of the aqueous dissolution of semicrystalline ...
2005-08-01 [Appl. Spectrosc. 59(8) , 976-85, (2005)] |
|
Solid phase extraction and high performance liquid chromatog...
1990-09-01 [Biomed. Chromatogr. 4(5) , 205-7, (1990)] |