FEBS Letters
1985-01-28
The specificity and kinetic properties of trypsin ethylated at the binding site.
R Ben Avraham, Y Shalitin
Index: FEBS Lett. 180(2) , 239-42, (1985)
Full Text: HTML
Abstract
Treatment of trypsin with triethyloxonium tetrafluoroborate at pH 8, 25 degrees C, results in abolition of binding to the enzyme of specific cationic substrates and inhibitors. The binding constant of soybean trypsin inhibitor to ethylated trypsin is 10000-fold smaller than to intact trypsin. However, the intrinsic ability of trypsin to recognize and react with nonspecific neutral substrates and inhibitors is not lost, and in several cases even considerably enhanced. Thus ethylated trypsin (Tret) resembles chymotrypsin in its behavior. Trypsin-like enzymes are also affected in a similar manner.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
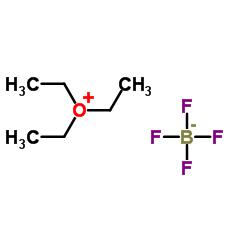 |
Triethyloxonium tetrafluoroborate
CAS:368-39-8 |
C6H15BF4O |
Related Articles:
More...
|
Determination of thiocyanate in saliva by headspace gas chro...
2015-06-26 [J. Chromatogr. A. 1400 , 124-30, (2015)] |
|
Method for activation and recycling of trityl resins.
2012-08-17 [J. Org. Chem. 77(16) , 7071-5, (2012)] |
|
Negative chemical ionization GC/MS determination of nitrite ...
2012-03-06 [Anal. Chem. 84(5) , 2592-6, (2012)] |
|
High yielding synthesis of N-ethyl dehydroamino acids.
2012-10-01 [Amino Acids 43(4) , 1643-52, (2012)] |
|
Some detoxification methods for N-nitrosamine-contaminated w...
1982-01-01 [IARC Sci. Publ. (41) , 151-7, (1982)] |