Negative chemical ionization GC/MS determination of nitrite and nitrate in seawater using exact matching double spike isotope dilution and derivatization with triethyloxonium tetrafluoroborate.
Enea Pagliano, Juris Meija, Ralph E Sturgeon, Zoltan Mester, Alessandro D'Ulivo
Index: Anal. Chem. 84(5) , 2592-6, (2012)
Full Text: HTML
Abstract
The alkylation of nitrite and nitrate by triethyloxonium tetrafluoroborate allows determination of their ethyl esters by headspace gas chromatography/mass spectrometry (GC/MS). In the present study, significant improvement in analytical performance is achieved using negative chemical ionization providing detection limits of 150 ng/L for NO(2)(-) and 600 ng/L for NO(3)(-), an order of magnitude better than those achieved using electron impact ionization. The derivatization procedure was optimized and alkaline conditions adopted to minimize conversion of nitrite to nitrate (determined to be 0.07% at 100 mg/L NO(2)(-)) and to avoid the exchange of oxygen between the analytes and the solvent (water). Quantitation entails use of isotopically enriched standards (N(18)O(2)(-) and (15)NO(3)(-)), which also permits monitoring of potential conversion from nitrite to nitrate during the analysis (double spike isotope dilution).
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
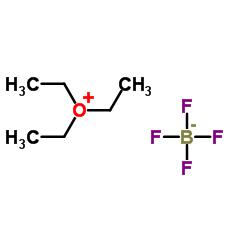 |
Triethyloxonium tetrafluoroborate
CAS:368-39-8 |
C6H15BF4O |
|
Determination of thiocyanate in saliva by headspace gas chro...
2015-06-26 [J. Chromatogr. A. 1400 , 124-30, (2015)] |
|
Method for activation and recycling of trityl resins.
2012-08-17 [J. Org. Chem. 77(16) , 7071-5, (2012)] |
|
The specificity and kinetic properties of trypsin ethylated ...
1985-01-28 [FEBS Lett. 180(2) , 239-42, (1985)] |
|
High yielding synthesis of N-ethyl dehydroamino acids.
2012-10-01 [Amino Acids 43(4) , 1643-52, (2012)] |
|
Some detoxification methods for N-nitrosamine-contaminated w...
1982-01-01 [IARC Sci. Publ. (41) , 151-7, (1982)] |