Liquid chromatographic analysis of amphetamine and related compounds in urine using solid-phase extraction and 3,5-dinitrobenzoyl chloride for derivatization.
R Herráez-Hernández, P Campíns-Falcó, A Sevillano-Cabeza
Index: J. Chromatogr. Sci. 35(4) , 169-75, (1997)
Full Text: HTML
Abstract
A chromatographic method for the analysis of amphetamine and related compounds in urine using 3,5-dinitrobenzoyl chloride (3,5-DNB) as a labeling reagent is presented. This assay is based on the employment of solid-phase extraction (SPE) cartridges for sample cleanup and derivatization. Experimental conditions are optimized for the simultaneous derivatization of ephedrine, norephedrine, pseudoephedrine, beta-phenylethylamine, amphetamine, methamphetamine, and 3-phenylpropylamine. The derivatives formed are separated in a LiChrospher 1000 RP18 (125 x 4-mm i.d., 5-microns film thickness) analytical column using a water-acetonitrile gradient elution and detected at 254 nm. Derivatization in C18 SPE disks is found to be the best option for analysis of urine samples; this method provides analyte conversions that are about 85-102% of those obtained by the analogous solution derivatization. Because the 3,5-DNB reagent is a strong pi-acid, the described method can be used in combination with a Pirkle-type donor column for chiral analysis. The practicality of the described approach is illustrated by determining amphetamine enantiomers using a Supelcosil LC-(S)-naphtylurea (250 x 4.6-mm i.d., 5-microns film thickness) column and a mobile phase of n-hexane-acetonitrile-ethyl acetate. Under these conditions, good linearity and reproducibility are observed over the 0.5-10 micrograms/ml concentration range; the limit of detection is 50 ng/mL.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
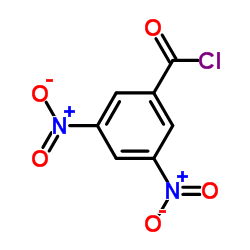 |
3,5-Dinitrobenzoyl chloride
CAS:99-33-2 |
C7H3ClN2O5 |
|
Refinements to the structure of graphite oxide: absolute qua...
2015-12-21 [Nanoscale 7 , 20256-66, (2015)] |
|
Liquid chromatographic determination of biogenic amines in f...
2000-06-09 [J. Chromatogr. A. 881(1-2) , 517-30, (2000)] |
|
Bombesin stimulates distinct time-dependent changes in the s...
1993-01-15 [Biochem. J. 289 , 487, (1993)] |
|
Common filaggrin gene mutations and risk of cervical cancer.
2015-02-01 [Acta Oncol. 54(2) , 217-23, (2015)] |
|
Synthetic analogues of netropsin and distamycin. IV. Synthes...
[Rocz. Akad. Med. Bialymst. 42(1) , 129-40, (1997)] |