| Structure | Name/CAS No. | Articles |
|---|---|---|
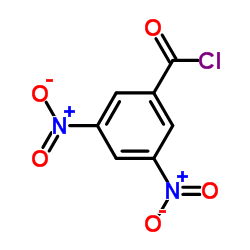 |
3,5-Dinitrobenzoyl chloride
CAS:99-33-2 |
A Markowska, D Bartulewicz, A Pućkowska, A Rózański
Index: Rocz. Akad. Med. Bialymst. 42(1) , 129-40, (1997)
Full Text: HTML
A carbocyclic analogue of distamycin was obtained, in which the N-methylpyrrole rings were substituted by disubstituted benzene rings. Additionally, N-chloro- or N-bromoacetyl groups, displaying alkylating properties, were introduced. The synthesis, starting from 3,5-dinitrobenzoyl chloride, consisted of five stages.
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
 |
3,5-Dinitrobenzoyl chloride
CAS:99-33-2 |
C7H3ClN2O5 |
|
Refinements to the structure of graphite oxide: absolute qua...
2015-12-21 [Nanoscale 7 , 20256-66, (2015)] |
|
Liquid chromatographic determination of biogenic amines in f...
2000-06-09 [J. Chromatogr. A. 881(1-2) , 517-30, (2000)] |
|
Bombesin stimulates distinct time-dependent changes in the s...
1993-01-15 [Biochem. J. 289 , 487, (1993)] |
|
Common filaggrin gene mutations and risk of cervical cancer.
2015-02-01 [Acta Oncol. 54(2) , 217-23, (2015)] |
|
HPLC analysis of polyamines and their acetylated derivatives...
1988-11-01 [Biomed. Chromatogr. 2(6) , 254-7, (1988)] |
Home | MSDS/SDS Database Search | Journals | Product Classification | Biologically Active Compounds | Selling Leads | About Us | Disclaimer
Copyright © 2026 ChemSrc All Rights Reserved