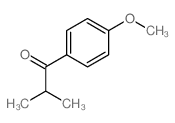
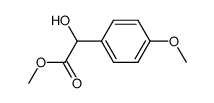
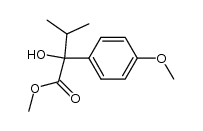
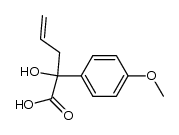
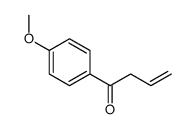
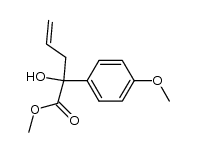
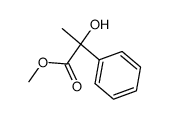
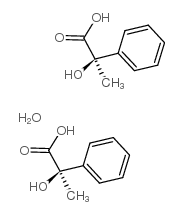
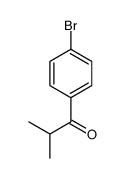
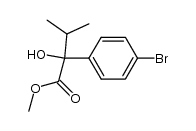
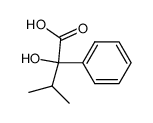
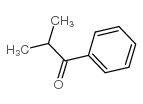
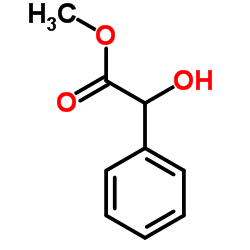
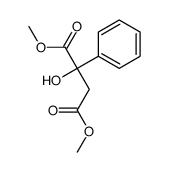
|
~88% |
|
~% |
|
~% |
|
~88% |
|
~% |
|
~% |
|
~% |
|
~79% |
|
~84% |
|
~% |
|
~% |
|
~53% |
|
~% |