| Structure | Name/CAS No. | Articles |
|---|---|---|
![(2S)-3,3'-Diphenyl[2,2'-binaphthalene]-1,1'-diol Structure](https://image.chemsrc.com/caspic/319/147702-14-5.png) |
(2S)-3,3'-Diphenyl[2,2'-binaphthalene]-1,1'-diol
CAS:147702-14-5 |
|
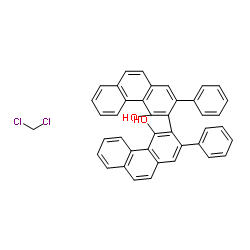 |
(S)-VAPOL
CAS:147702-15-6 |
|
 |
3,3'-Diphenyl-2,2'-binaphthalene-1,1'-diol
CAS:147702-13-4 |
|
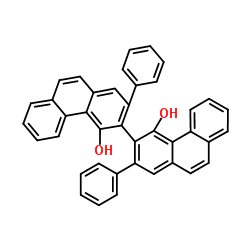 |
2,2'-Diphenyl-3,3'-biphenanthrene-4,4'-diol
CAS:147702-16-7 |