| Structure | Name/CAS No. | Articles |
|---|---|---|
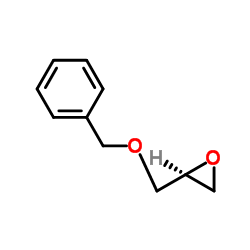 |
(S)-(+)-Benzyl glycidyl ether
CAS:16495-13-9 |
| Structure | Name/CAS No. | Articles |
|---|---|---|
 |
(S)-(+)-Benzyl glycidyl ether
CAS:16495-13-9 |