On the mechanism of the Skraup-Doebner-Von Miller quinoline synthesis.
Scott E Denmark, Srikanth Venkatraman
Index: J. Org. Chem. 71(4) , 1668-76, (2006)
Full Text: HTML
Abstract
The mechanism of the formation of substituted quinolines from anilines and alpha,beta-unsaturated ketones has been studied by the use of 13C-labeled ketones in cross-over experiments. In the reaction of doubly labeled 13C(2,4) mesityl oxide, a 100% scrambling of the label in the quinoline product was observed, whereas only a small (5-10%) amount of the starting mesityl oxide showed scrambling of the label. Similarly, the reaction of triply labeled pulegone clearly shows that the label in the product is 100% scrambled, whereas the label in the starting pulegone is retained. On the basis of these studies, a mechanistic pathway for the Skraup quinoline synthesis is proposed that involves a fragmentation-recombination mechanism. The aniline component condenses with the alpha,beta-unsaturated ketone initially in a conjugate fashion, followed by a fragmentation to the corresponding imine and the ketone itself. These fragments recombine to form the quinoline product.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
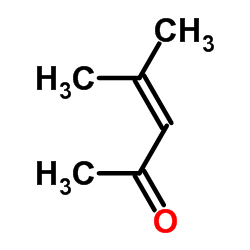 |
Mesityl oxide
CAS:141-79-7 |
C6H10O |
|
Synthesis of modified homo-N-nucleosides from the reactions ...
2009-01-01 [Bioorg. Med. Chem. Lett. 19 , 6433-6, (2009)] |
|
The strongest isolable acid.
2004-10-11 [Angew. Chem. Int. Ed. Engl. 43(40) , 5352-5, (2004)] |
|
Preparation and evaluation of an imidazole-coated capillary ...
2000-11-03 [J. Chromatogr. A. 897(1-2) , 383-92, (2000)] |
|
Refined Synthesis of 2,3,4,5-Tetrahydro-1,3,3-trimethyldipyr...
2009-03-01 [Org. Process Res. Dev. 9(5) , 651-659, (2005)] |
|
Two-dimensional capillary electrophoresis using tangentially...
2007-06-22 [J. Chromatogr. A. 1154(1-2) , 454-9, (2007)] |