| Structure | Name/CAS No. | Articles |
|---|---|---|
 |
Isoniazid
CAS:54-85-3 |
|
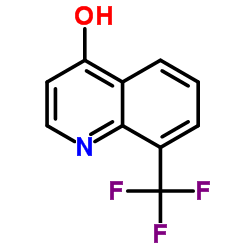 |
8-(Trifluoromethyl)-4-quinolinol
CAS:23779-96-6 |
|
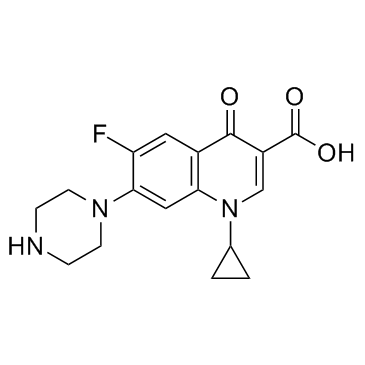 |
Ciprofloxacin
CAS:85721-33-1 |
|
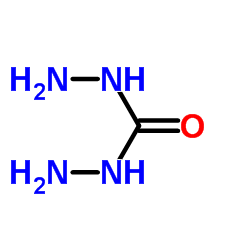 |
carbohydrazide
CAS:497-18-7 |