| Structure | Name/CAS No. | Articles |
|---|---|---|
 |
Caryophyllene oxide
CAS:1139-30-6 |
|
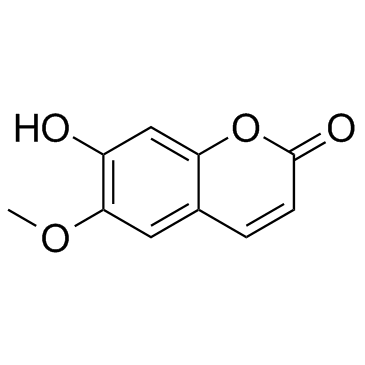 |
Scopoletin
CAS:92-61-5 |
|
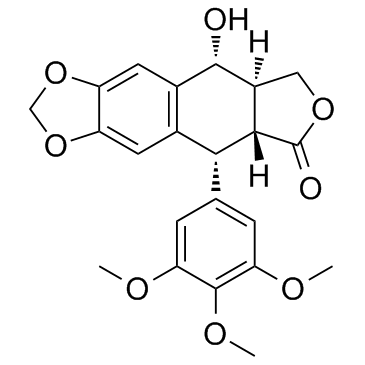 |
Podophyllotoxin
CAS:518-28-5 |