| Structure | Name/CAS No. | Articles |
|---|---|---|
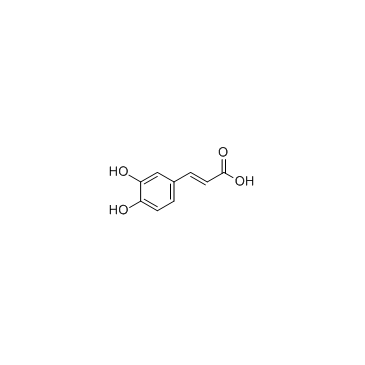 |
Caffeic acid
CAS:331-39-5 |
|
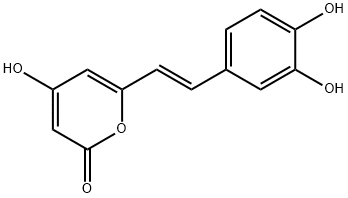 |
HISPIDIN
CAS:555-55-5 |
|
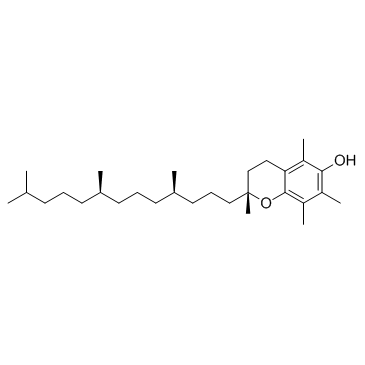 |
DL-alpha-Tocopherol
CAS:59-02-9 |