| Structure | Name/CAS No. | Articles |
|---|---|---|
 |
Acetyl chloride
CAS:75-36-5 |
|
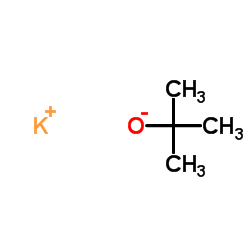 |
potassium t-butoxide
CAS:865-47-4 |
|
 |
2,2,2-Trifluoroacetamide
CAS:354-38-1 |