| Structure | Name/CAS No. | Articles |
|---|---|---|
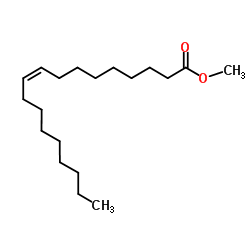 |
Methyl oleate
CAS:112-62-9 |
|
 |
Oxidase choline
CAS:9028-67-5 |
|
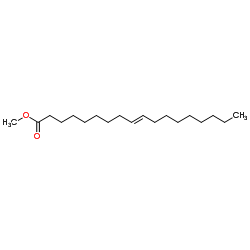 |
Methyl (9E)-9-octadecenoate
CAS:1937-62-8 |
|
 |
Glyceryl monooleate
CAS:25496-72-4 |