| Structure | Name/CAS No. | Articles |
|---|---|---|
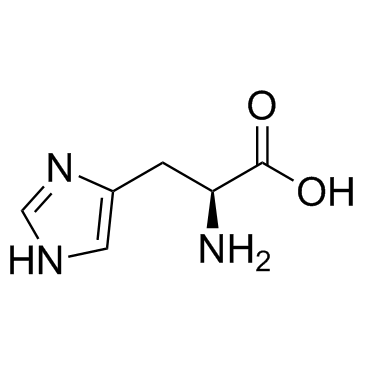 |
L-Histidine
CAS:71-00-1 |
|
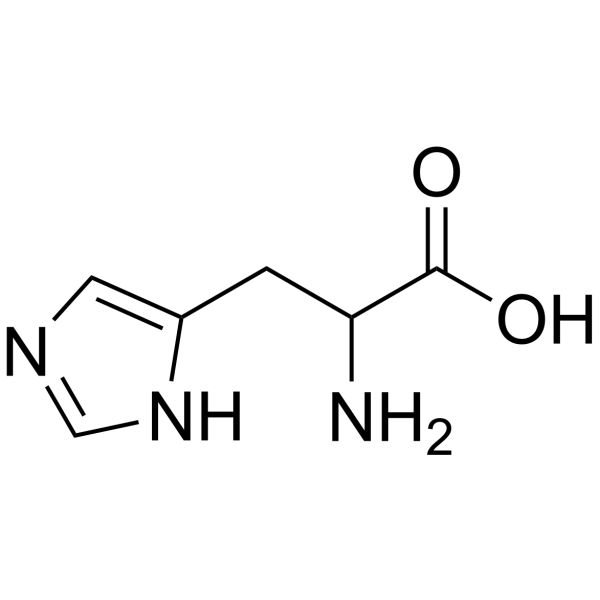 |
DL-Histidine
CAS:4998-57-6 |
|
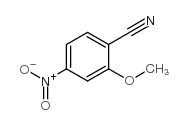 |
2-Methoxy-4-nitrobenzonitrile
CAS:101084-96-2 |