| Structure | Name/CAS No. | Articles |
|---|---|---|
 |
Furosemide
CAS:54-31-9 |
|
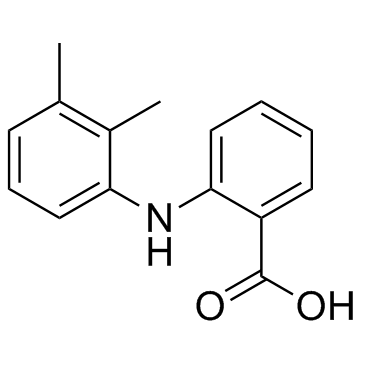 |
Mefenamic acid
CAS:61-68-7 |
|
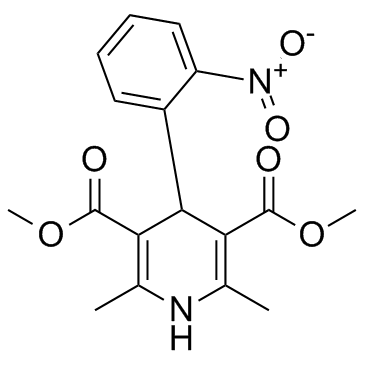 |
Nifedipine
CAS:21829-25-4 |
|
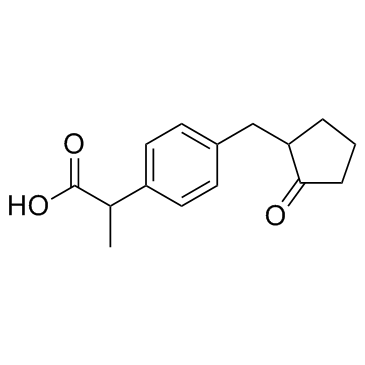 |
Loxoprofen
CAS:68767-14-6 |