| Structure | Name/CAS No. | Articles |
|---|---|---|
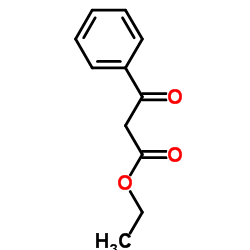 |
Ethyl 3-oxo-3-phenylpropanoate
CAS:94-02-0 |
|
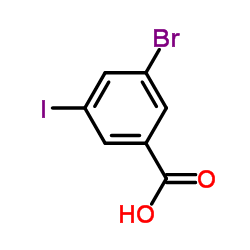 |
3-Bromo-5-iodobenzoic acid
CAS:188815-32-9 |