Organic Letters
2011-05-06
A practical and convenient fluorination of 1,3-dicarbonyl compounds using aqueous HF in the presence of iodosylbenzene.
Tsugio Kitamura, Satoshi Kuriki, Mohammad Hasan Morshed, Yuji Hori
Index: Org. Lett. 13 , 2392-2394, (2011)
Full Text: HTML
Abstract
A simple, practical, and convenient fluorination of 1,3-dicarbonyl compounds was achieved by direct use of aqueous hydrofluoric acid and iodosylbenzene (PhIO). The reaction of ethyl benzoylacetate with the reagent system of aqueous HF and PhIO in CH(2)Cl(2) gave ethyl 2-fluoro-2-benzolyacetate in 98% yield. Other 1,3-dicarbonyl compounds including β-keto esters and 1,3-diketones underwent the fluorination reaction to give the corresponding fluorinated products in good yields.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
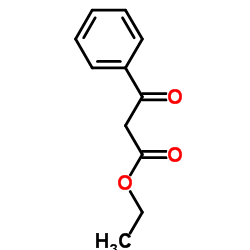 |
Ethyl 3-oxo-3-phenylpropanoate
CAS:94-02-0 |
C11H12O3 |
Related Articles:
More...
|
Reaction of iodonium ylides of 1,3-dicarbonyl compounds with...
2012-01-01 [Molecules 17(6) , 6625-32, (2012)] |
|
A short synthesis of the triazolopyrimidine antibiotic essra...
2010-11-29 [J. Nat. Prod. 73(11) , 1938-9, (2010)] |
|
Free radical reaction between 2-benzoyl-1,4-benzoquinones an...
2009-10-07 [Org. Biomol. Chem. 7(19) , 4074-81, (2009)] |
|
3-Amino-8-hydroxy-4-imino-6-methyl-5-phenyl-4,5-dihydro-3H-c...
2012-01-01 [Molecules 17(10) , 11538-53, (2012)] |
|
Asymmetric synthesis of both enantiomers of fluoxetine via m...
[Tetrahedron 48(33) , 6769-76, (1992)] |