Identification of functional domains of the human glutamate transporters EAAT1 and EAAT2.
A D Mitrovic, S G Amara, G A Johnston, R J Vandenberg
Index: J. Biol. Chem. 273(24) , 14698-706, (1998)
Full Text: HTML
Abstract
Glutamate transporters serve the important function of mediating removal of glutamate released at excitatory synapses and maintaining extracellular concentrations below excitotoxic levels. Excitatory amino acid transporter subtypes EAAT1 and EAAT2 have a high degree of sequence homology and similar predicted topology and yet display a number of functional differences. Several recombinant chimeric transporters were generated to identify domains that contribute to functional differences between EAAT1 and EAAT2. Wild-type transporters and chimeric transporters were expressed in Xenopus laevis oocytes, and electrogenic transport was studied under voltage clamp conditions. The differential sensitivity of EAAT1 and EAAT2 to transport blockers, kainate, threo-3-methylglutamate, and (2S, 4R)-4-methylglutamate as well as L-serine-O-sulfate transport and chloride permeability were employed to characterize chimeric transporters. One particular region, transmembrane domains 9 and 10, plays an important role in defining these functional differences. The intracellular carboxyl-terminal region may also play a minor role in conferring an effect on chloride permeability. This study provides important insight into the identification of functional domains that determine differences among glutamate transporter subtypes.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
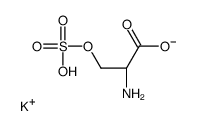 |
L-Serine O-sulfate potassium salt
CAS:17436-02-1 |
C3H6KNO6S |
|
Sulphur-containing amino acids are agonists for group 1 meta...
1998-01-01 [Neuropharmacology 37 , 277-287, (1998)] |
|
Effects of glutamate transporter inhibitors on the antitumor...
2002-12-01 [Clin. Cancer Res. 8(12) , 3943-7, (2002)] |
|
The mechanism of high-yielding chiral syntheses catalysed by...
1996-08-15 [Eur. J. Biochem. 240(1) , 150-5, (1996)] |
|
Characterization of Na+-coupled glutamate/aspartate transpor...
2001-07-01 [J. Membr. Biol. 182(1) , 17-30, (2001)] |
|
Purification and characterization of human brain serine race...
2009-01-01 [Protein Pept. Lett. 16(2) , 201-6, (2009)] |