The mechanism of high-yielding chiral syntheses catalysed by wild-type and mutant forms of aspartate aminotransferase.
R Contestabile, R A John
Index: Eur. J. Biochem. 240(1) , 150-5, (1996)
Full Text: HTML
Abstract
The ability of aspartate aminotransferase to catalyse beta-elimination of alpha-amino acids that have a good leaving group at C beta has been exploited in the synthesis of novel amino acids by the inclusion of appropriate nucleophiles as co-substrates. Two compounds, L-serine O-sulphate and 3-chloro-L-alanine, were used as beta-elimination substrates. Nucleophiles used successfully as co-substrates were thiosulphate, 2-mercaptoethanol, mercaptoacetate and aminoethylthiopseudourea. The synthesis achieved using serine O-sulphate and thiosulphate was found to produce sulphocysteine with a yield of 70%. Circular dichroism demonstrated that the compound was a single enantiomer and, therefore, that nucleophilic addition had taken place on the enzyme. The initial rate of synthesis was 10% of the rate at which the enzyme catalyses its normal transamination reaction. The synthetic reaction was accompanied by minor side reactions that led to small amounts of additional amino acid and oxo acid products through partitions of the main reaction at two stages in the mechanism. By mutating Arg292, which is the residue that binds the distal carboxyl group of natural substrates, the wild-type enzyme was converted to a form that could discriminate completely between serine O-sulphate and chloroalanine as beta-eliminating substrate. Similar alterations in nucleophile cosubstrate specificity were also observed. Whereas, for example, the wild-type enzyme catalysed syntheses between 3-chloroalanine and either mercaptoethanol or mercaptoacetate with equal facility, the Arg292Asp enzyme showed complete preference for mercaptoethanol. The system should be of general use in the synthesis of novel amino acids as single enantiomers with potentially interesting biological activities.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
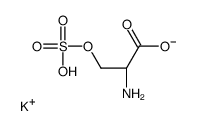 |
L-Serine O-sulfate potassium salt
CAS:17436-02-1 |
C3H6KNO6S |
|
Sulphur-containing amino acids are agonists for group 1 meta...
1998-01-01 [Neuropharmacology 37 , 277-287, (1998)] |
|
Effects of glutamate transporter inhibitors on the antitumor...
2002-12-01 [Clin. Cancer Res. 8(12) , 3943-7, (2002)] |
|
Characterization of Na+-coupled glutamate/aspartate transpor...
2001-07-01 [J. Membr. Biol. 182(1) , 17-30, (2001)] |
|
Purification and characterization of human brain serine race...
2009-01-01 [Protein Pept. Lett. 16(2) , 201-6, (2009)] |
|
Metabolism of sulfur-containing amino acids in the dermatoph...
1985-01-01 [J. Basic Microbiol. 25(2) , 111-8, (1985)] |