| Structure | Name/CAS No. | Articles |
|---|---|---|
 |
4-Chloro-3-(trifluoromethyl)phenylisocyanate
CAS:327-78-6 |
|
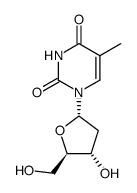 |
ALPHA-THYMIDINE
CAS:4449-43-8 |
|
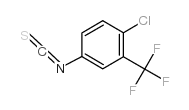 |
4-chloro-3-(trifluoromethyl)phenyl isothiocyanate
CAS:23163-86-2 |