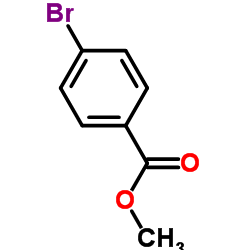
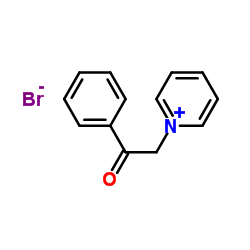
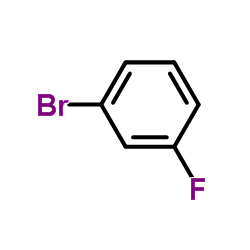
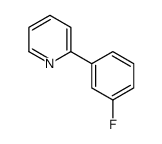
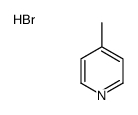
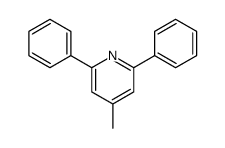
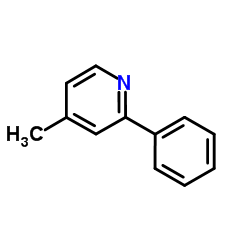
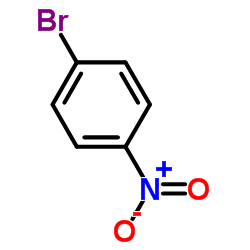
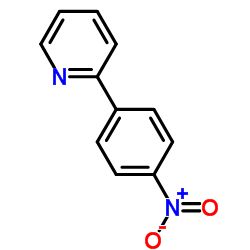
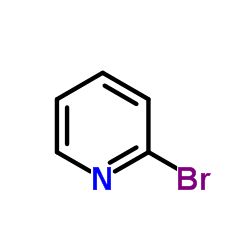
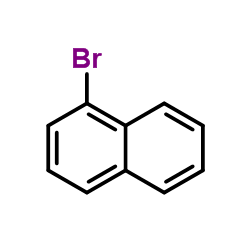
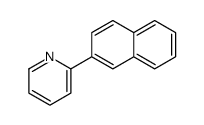
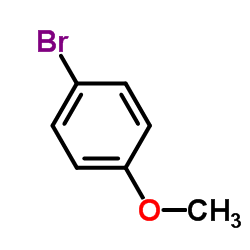
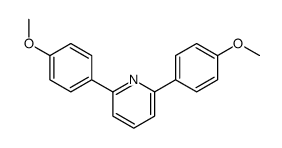
|
~71% |
|
~41% |
|
~36% |
|
~75% |
|
~%
Detail
|
|
~62% |
|
~82% |
|
~35% |
|
~46% |