[Randomized double-blind comparative study of minaprine (200mg/j) and of placebo on memory loss].
H Allain, S Belliard, A Lieury, G Menard, A Patat, F Le Coz, J M Gandon
Index: Therapie. 51(2) , 155-62, (1996)
Full Text: HTML
Abstract
Thirty five subjects (age: 45-69 years) with subjective memory loss, without any other neuropsychiatric or somatic disease, were recruited in a phase II study. This double blind randomized versus placebo controlled study compared the effects of minaprine (200 mg/d) with placebo, in two parallel groups, during 2 months, on memory, attention and vigilance. Three psychometric tests were the main criteria of assessment: a standardized battery of memory tests (SM 5), the dual-coding test, the analysis of choice reaction times (CRT) and the critical flicker fusion point (CFF). A positive effect of minaprine was detected on words delayed recall (p = 0.028) and immediate recognition of words (p = 0.049). The global clinical tests (CGI, MacNair scale) were not statistically modified. Tolerability of minaprine and placebo were comparable. A positive pharmacodynamic activity on mnemonic performance is thus demonstrated in favour of minaprine (200 mg/d) in this specific population characterized by a memory complaint. These results would lead to a phase III study in which the main criteria would be global scales in order to confirm the clinical reliability of the present results.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
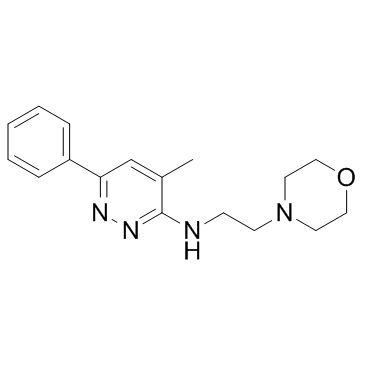 |
Minaprine
CAS:25905-77-5 |
C17H22N4O |
|
Multicentric double-blind study comparing efficacy and safet...
1995-01-01 [Neuropsychobiology 31(2) , 68-75, (1995)] |
|
Computerised psychomotor performance testing: a comparative ...
1993-10-01 [Br. J. Clin. Pharmacol. 36(4) , 376-9, (1993)] |
|
Cholinomimetic activity of minaprine is related to the ameli...
1992-07-01 [Physiol. Behav. 52(1) , 141-7, (1992)] |
|
Minaprine facilitates acquisition and retrieval of an active...
1993-06-01 [Pharmacol. Biochem. Behav. 45(2) , 481-5, (1993)] |
|
Effects of subchronic minaprine on dopamine release in the v...
1994-01-17 [Neurosci. Lett. 166(1) , 69-72, (1994)] |