| Structure | Name/CAS No. | Articles |
|---|---|---|
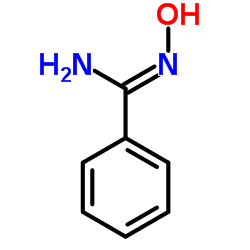 |
N-Hydroxybenzenecarboximidamide
CAS:613-92-3 |
|
![4-[5-(4-aminophenyl)-1,3,4-oxadiazol-2-yl]aniline Structure](https://image.chemsrc.com/caspic/483/2425-95-8.png) |
4-[5-(4-aminophenyl)-1,3,4-oxadiazol-2-yl]aniline
CAS:2425-95-8 |
|
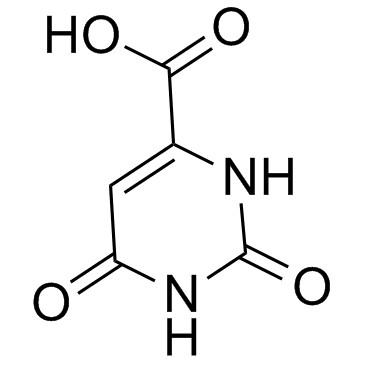 |
Orotic acid
CAS:65-86-1 |
|
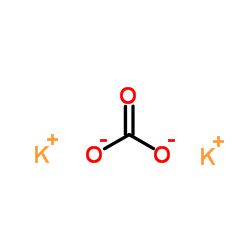 |
Potassium carbonate
CAS:584-08-7 |