Inactivation of arginine esterase E-I of Bitis gabonica venom by irreversible inhibitors including a water-soluble carbodiimide, a chloromethyl ketone and isatoic anhydride.
P S Gravett, C C Viljoen, M M Oosthuizen
Index: Int. J. Biochem. 23(10) , 1101-10, (1991)
Full Text: HTML
Abstract
1. Esterase E-I from Bitis gabonica was inactivated with irreversible inhibitors which included studies with a water-soluble carbodiimide, an affinity labelling peptide and a mechanism-based inactivator. 2. The reaction with 1-ethyl-3(3-dimethylaminopropyl)-carbodiimide was biphasic and the dominant part followed saturation kinetics. At pH 5.5 a rate constant of 0.4 min-1 for inactive enzyme formation was calculated and a dissociation constant (Ki) of 0.2 M for the enzyme-inhibitor complex. 3. Inactivation with D-Phe-Pro-Arg-chloromethyl ketone indicated a two-step mechanism, for which the reaction parameters at pH 8.0 were determined. The Ki value was 0.2 microM and the inactivation rate was 2.5 min-1. 4. With isatoic anhydride pseudo-first-order kinetics was observed. At pH 8.0 a rate constant of 0.9 min-1 and a Ki of 2.0 mM were obtained. The inactivation of the enzyme was found to be governed by a group in the enzyme showing a pK value of 7.3.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
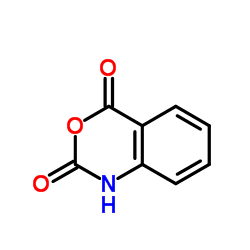 |
Isatoic anhydride
CAS:118-48-9 |
C8H5NO3 |
|
Hydrogen-bonding in 2-aminobenzoyl-alpha-chymotrypsin formed...
2002-01-04 [ChemBioChem. 3(1) , 68-75, (2002)] |
|
Palladium-catalyzed decarboxylative coupling of isatoic anhy...
2011-11-18 [Org. Lett. 13 , 6114-6117, (2011)] |
|
1,3-Dipolar cycloaddition-decarboxylation reactions of an az...
2011-02-04 [Org. Lett. 13(3) , 486-9, (2011)] |
|
Suicide enzyme inactivators.
1983-01-01 [Basic Life Sci. 25 , 287-305, (1983)] |
|
Synthesis of 2,3-dihydroquinazolin-4(1H)-ones by three-compo...
2010-09-13 [J. Comb. Chem. 12(5) , 643-6, (2010)] |