Substituted isatoic anhydrides: selective inactivators of trypsin-like serine proteases.
M H Gelb, R H Abeles
Index: J. Med. Chem. 29(4) , 585-9, (1986)
Full Text: HTML
Abstract
Derivatives of isatoic anhydride were prepared and tested as inhibitors of serine proteases. A number of isatoic anhydrides with positively charged substituents irreversibly inactivated several trypsin-like enzymes and preferentially inactivated trypsin over chymotrypsin. Further selectivity was obtained by introduction of an aromatic group on the N-1 position of isatoic anhydride. 7-(Aminomethyl)-1-benzylisatoic anhydride was prepared and was a selective inactivator of thrombin; thus it is possible to prepare derivatives of isatoic anhydride that are highly enzyme selective without attaching peptide recognition structures.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
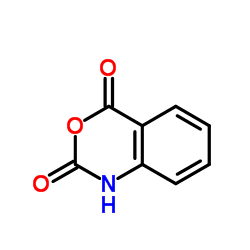 |
Isatoic anhydride
CAS:118-48-9 |
C8H5NO3 |
|
Hydrogen-bonding in 2-aminobenzoyl-alpha-chymotrypsin formed...
2002-01-04 [ChemBioChem. 3(1) , 68-75, (2002)] |
|
Palladium-catalyzed decarboxylative coupling of isatoic anhy...
2011-11-18 [Org. Lett. 13 , 6114-6117, (2011)] |
|
1,3-Dipolar cycloaddition-decarboxylation reactions of an az...
2011-02-04 [Org. Lett. 13(3) , 486-9, (2011)] |
|
Suicide enzyme inactivators.
1983-01-01 [Basic Life Sci. 25 , 287-305, (1983)] |
|
Synthesis of 2,3-dihydroquinazolin-4(1H)-ones by three-compo...
2010-09-13 [J. Comb. Chem. 12(5) , 643-6, (2010)] |