Total synthesis of (+)-cymbodiacetal: a re-evaluation of the biomimetic route.
Maliha Uroos, William Lewis, Alexander J Blake, Christopher J Hayes
Index: J. Org. Chem. 75(24) , 8465-70, (2010)
Full Text: HTML
Abstract
A total synthesis of (+)-cymbodiacetal (1) has been completed from (R)-(+)-limonene oxide using a hetero-Diels-Alder cycloaddition as a key step. The key Diels-Alder cycloaddition proceeds with endo-selectivity (2:1, endo/exo) in quantitative yield, and the two diastereomeric spirochroman products are isolable, stable products. Furthermore, the exo- and the endo-hetero-Diels-Alder cycloaddition products (2 and 7) can be oxidized with m-CPBA to produce (+)-cymbodiacetal (1) and the C(2)-symmetric bis-hemiacetal structure 8, respectively. The isomeric hemiacetal 9 is produced in both oxidation reactions. The structures of (+)-cymbodiacetal (1), its C(2)-symmetric diastereoisomer 8, and the isomeric hemiacetal 9 were confirmed using X-ray crystallography.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
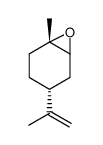 |
(+)-Trans-limonene1,2-epoxide
CAS:6909-30-4 |
C10H16O |
|
Catalytic mechanism of limonene epoxide hydrolase, a theoret...
2005-10-19 [J. Am. Chem. Soc. 127(41) , 14339-47, (2005)] |
|
QM/MM study of the mechanism of enzymatic limonene 1,2-epoxi...
2012-02-01 [Biochim. Biophys. Acta 1824(2) , 263-8, (2012)] |
|
Metabolism of limonene-1,2-epoxide in the rat.
1980-12-01 [Xenobiotica 10(12) , 859-61, (1980)] |
|
Indoloquinazoline alkaloids from Euodia rutaecarpa and their...
2011-10-01 [J. Asian Nat. Prod. Res. 13(11) , 977-83, (2011)] |
|
The structures of alpha 2u-globulin and its complex with a h...
1999-04-01 [Acta Crystallogr. D Biol. Crystallogr. 55(Pt 4) , 753-62, (1999)] |