| Structure | Name/CAS No. | Articles |
|---|---|---|
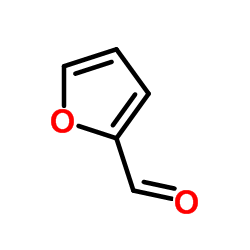 |
Furfural
CAS:98-01-1 |
|
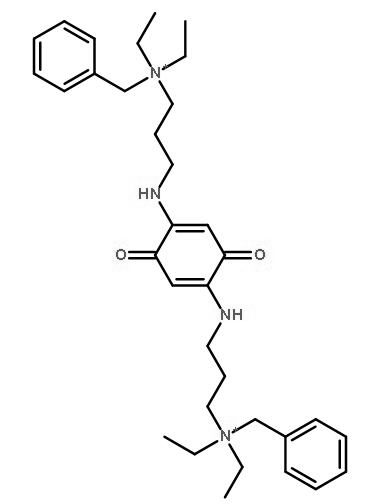 |
Endo-1,4-β-xylanase
CAS:9025-57-4 |
|
 |
β-Glucosidase
CAS:9001-22-3 |
|
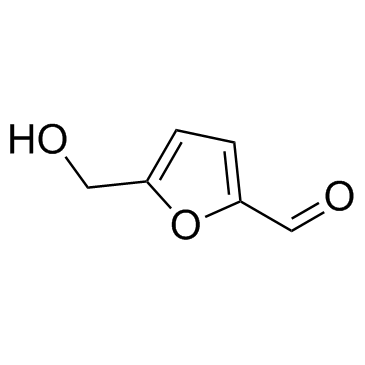 |
5-hydroxymethylfurfural
CAS:67-47-0 |