The acid-tolerant L-arabinose isomerase from the mesophilic Shewanella sp. ANA-3 is highly active at low temperatures.
Moez Rhimi, Goran Bajic, Rimeh Ilhammami, Samira Boudebbouze, Emmanuelle Maguin, Richard Haser, Nushin Aghajari
Index: Microb. Cell Fact. 10 , 96, (2011)
Full Text: HTML
Abstract
L-arabinose isomerases catalyse the isomerization of L-arabinose into L-ribulose at insight biological systems. At industrial scale of this enzyme is used for the bioconversion of D-galactose into D-tagatose which has many applications in pharmaceutical and agro-food industries. The isomerization reaction is thermodynamically equilibrated, and therefore the bioconversion rates is shifted towards tagatose when the temperature is increased. Moreover, to prevent secondary reactions it will be of interest to operate at low pH. The profitability of this D-tagatose production process is mainly related to the use of lactose as cheaper raw material. In many dairy products it will be interesting to produce D-tagatose during storage. This requires an efficient L-arabinose isomerase acting at low temperature and pH values.The gene encoding the L-arabinose isomerase from Shewanella sp. ANA-3 was cloned and overexpressed in Escherichia coli. The purified protein has a tetrameric arrangement composed by four identical 55 kDa subunits. The biochemical characterization of this enzyme showed that it was distinguishable by its maximal activity at low temperatures comprised between 15-35°C. Interestingly, this biocatalyst preserves more than 85% of its activity in a broad range of temperatures from 4.0 to 45°C. Shewanella sp. ANA-3 L-arabinose isomerase was also optimally active at pH 5.5-6.5 and maintained over 80% of its activity at large pH values from 4.0 to 8.5. Furthermore, this enzyme exhibited a weak requirement for metallic ions for its activity evaluated at 0.6 mM Mn2+. Stability studies showed that this protein is highly stable mainly at low temperature and pH values. Remarkably, T268K mutation clearly enhances the enzyme stability at low pH values. Use of this L-arabinose isomerase for D-tagatose production allows the achievement of attractive bioconversion rates of 16% at 4°C and 34% at 35°C.Here we reported the purification and the biochemical characterization of the novel Shewanella sp. ANA-3 L-arabinose isomerase. Determination of the biochemical properties demonstrated that this enzyme was highly active at low temperatures. The generated T268K mutant displays an increase of the enzyme stability essentially at low pH. These features seem to be very attractive for the bioconversion of D-galactose into D-tagatose at low temperature which is very interesting from industrial point of view.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
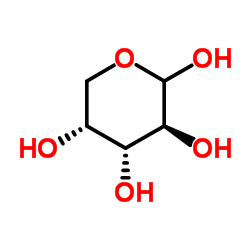 |
D-Arabinose
CAS:10323-20-3 |
C5H10O5 | |
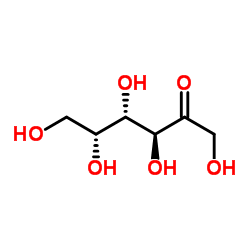 |
D-(-)-Tagatose
CAS:87-81-0 |
C6H12O6 |
|
Rehmannia glutinosa (Gaertn.) DC. polysaccharide ameliorates...
2015-04-22 [J. Ethnopharmacol. 164 , 229-38, (2015)] |
|
Structure and bioactivity of a polysaccharide extracted from...
2015-01-01 [Int. J. Biol. Macromol. 72 , 664-72, (2014)] |
|
Detoxification of biomass hydrolysates with nucleophilic ami...
2015-06-01 [Bioresour. Technol. 186 , 106-13, (2015)] |
|
Antioxidant and antiproliferative activities of polysacchari...
2015-08-01 [Food Funct. 6 , 2598-606, (2015)] |
|
Structural features of dilute acid, steam exploded, and alka...
2015-06-25 [Carbohydr. Polym. 124 , 265-73, (2015)] |