| Structure | Name/CAS No. | Articles |
|---|---|---|
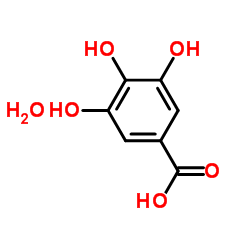 |
Gallic acid hydrate
CAS:5995-86-8 |
|
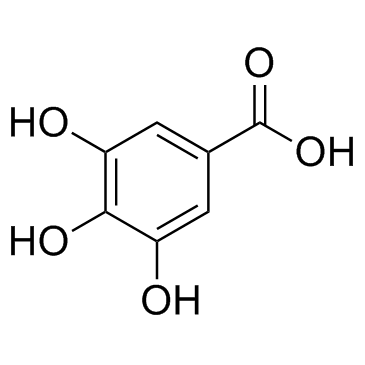 |
Gallic acid
CAS:149-91-7 |
|
 |
Syringic acid
CAS:530-57-4 |
|
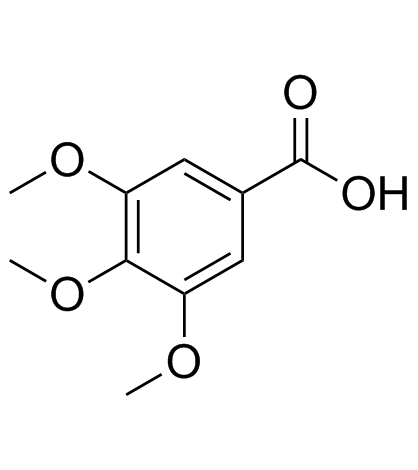 |
Trimethylgallic acid
CAS:118-41-2 |
|
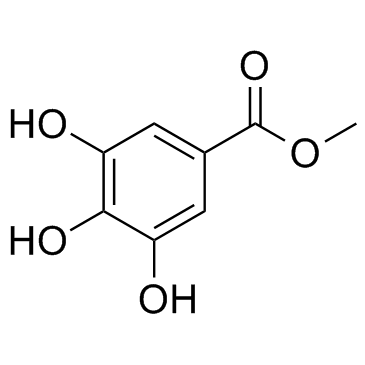 |
Methyl gallate
CAS:99-24-1 |