| Structure | Name/CAS No. | Articles |
|---|---|---|
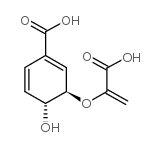 |
Chorismic acid, from Enterobacter aerogenes
CAS:617-12-9 |
|
 |
Pyruvate Oxidase
CAS:9001-96-1 |