| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
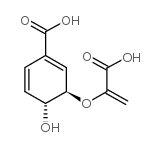 |
分支酸 来源于产气肠杆菌
CAS:617-12-9 |
|
 |
丙酮酸氧化酶
CAS:9001-96-1 |
| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
 |
分支酸 来源于产气肠杆菌
CAS:617-12-9 |
|
 |
丙酮酸氧化酶
CAS:9001-96-1 |