In vitro and in vivo effects of easily administered, low-toxic retinoid and phenylacetate compounds on human neuroblastoma cells.
N Sidell, M Pasquali, S Malkapuram, A B Barua, T Wanichkul, R K Wada
Index: Br. J. Cancer 89(2) , 412-9, (2003)
Full Text: HTML
Abstract
We have investigated the effects of the low-toxic retinoid, all-trans retinoyl beta-glucuronide (RAG) alone and in combination with the phenylacetate (PA) derivative 4-chloro-phenylacetate (4-CPA) on the human neuroblastoma cell line, LA-N-5. In vitro studies demonstrated that RAG and 4-CPA treatments alone showed differentiation-inducing activity on LA-N-5 cells, with 4-CPA found to be about three-fold more potent than the PA parent compound in inducing morphologic differentiation and growth inhibition. As previously reported for retinoic acid (RA) and PA, RAG and 4-CPA were significantly more effective in their antiproliferative effects on the cells than either agent alone. Pharmacologic studies of 4-CPA in mice demonstrated that blood plasma levels reached peak concentrations 4 h after bolus administration of the compound and showed slow clearance characteristics with an apparent half-life of 4-8 h. As opposed to PA, 4-CPA was found to be essentially odourless and readily consumed in drinking water, giving rise to steady-state blood plasma levels of 4-CPA in the near mM range. Continuous consumption of 4-CPA in this manner for up to 5 months demonstrated no apparent adverse effects on the mice. Long-term RAG- and/or 4-CPA-treatment of nude mice injected with LA-N-5 cells demonstrated that both compounds alone exhibit potent antitumour activity. Together, RAG plus 4-CPA was the most effective treatment for inhibiting established tumour growth. In contrast, 4-CPA alone was equally as effective as the combination for preventing tumour development. The potent in vivo antitumour effects of 4-CPA could not be accounted for by the known ability of PA compounds to induce expression of the RA nuclear receptor beta (RARbeta) suppressor gene. Taken together, these findings demonstrate the possibility that RAG and/or 4-CPA may serve as effective, less-toxic alternatives to 13-cis RA, which is presently being utilised for nb therapy.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
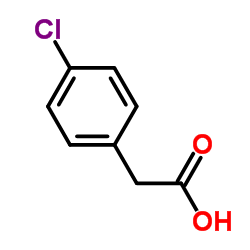 |
4-Chlorophenylacetic acid
CAS:1878-66-6 |
C8H7ClO2 |
|
Novel Chryseobacterium sp. PYR2 degrades various organochlor...
2015-09-15 [J. Environ. Manage. 161 , 350-7, (2015)] |
|
Degradation of 4-chlorophenylacetic acid by a Pseudomonas sp...
1981-04-01 [J. Bacteriol. 146(1) , 64-8, (1981)] |
|
Inhibition of estrogen-induced mammary tumor formation in MM...
2007-06-28 [Cancer Lett. 251(2) , 302-10, (2007)] |
|
Metabolomics reveals that aldose reductase activity due to A...
2015-07-01 [J. Viral Hepat. 22 , 617-24, (2015)] |
|
Oxidation and dehalogenation of 4-chlorophenylacetate by a t...
1984-11-01 [J. Bacteriol. 160(2) , 618-21, (1984)] |