| Structure | Name/CAS No. | Articles |
|---|---|---|
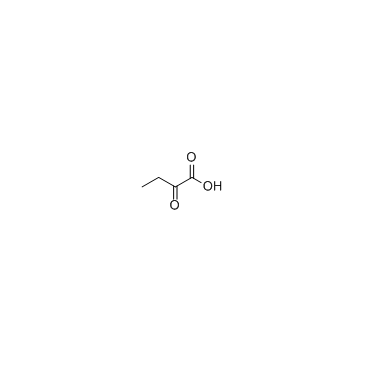 |
2-Oxobutyric acid
CAS:600-18-0 |
|
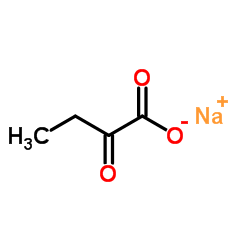 |
Sodium 2-oxobutanoate
CAS:2013-26-5 |
|
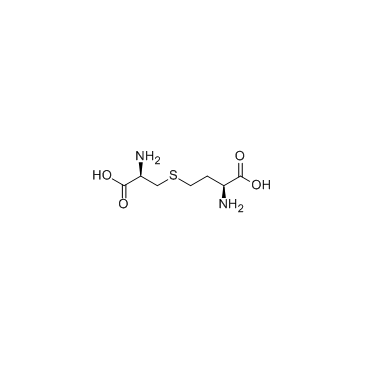 |
L-Cystathionine
CAS:56-88-2 |