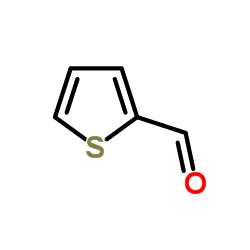
|
~85% |
|
~20% |
|
~21% |
|
~77% |
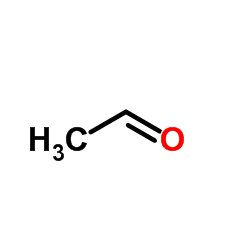
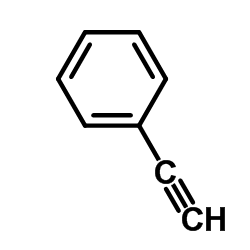
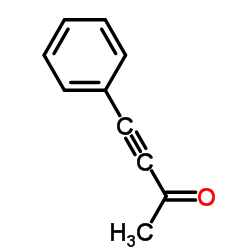

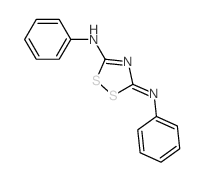


![N-[5-acetyl-3,4-diphenyl-1,3-thiazol-2(3H)-ylidene]-N'-phenylthiourea Structure](https://image.chemsrc.com/caspic/403/1159126-30-3.png)