Enantioselective analysis of glufosinate using precolumn derivatization with (+)-1-(9-fluorenyl)ethyl chloroformate and reversed-phase liquid chromatography.
Yasushi Hori, Manami Fujisawa, Kenji Shimada, Mitsuru Sato, Masao Honda, Yasuo Hirose
Index: J. Chromatogr. B. Analyt. Technol. Biomed. Life Sci. 2nd ed., 776 , 191, (2002)
Full Text: HTML
Abstract
We have developed a new analytical method to quantify the DL-homoalanine-4-yl(methyl)phosphinate (DL-GLUF) enantiomers in biological specimens using a reversed-phase high-performance liquid chromatography system with a fluorescence detection system. The derivatization of DL-GLUF enantiomers with (+)-1-(9-fluorenyl)ethyl chloroformate was carried out under mild conditions (40 degrees C for 30 min) without inducing racemization. The lower limit of quantitation was 0.01 microg/ml for both D-GLUF and L-GLUF, and the detection limit was 5 ng/ml. When DL-GLUF enantiomers were added to serum to produce concentrations between 0.1 and 100 microg/ml, the mean recovery rate was at least 93.8%. The recovery rate from urine was also satisfactory.Copyright 2002 Elsevier Science B.V.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
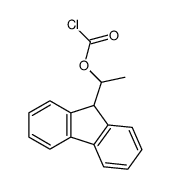 |
(+)-1-(9-fluorenyl)ethyl chloroformate
CAS:107474-79-3 |
C16H13ClO2 |
|
Enantioselective analysis of D- and L-amino acids from mouse...
2015-12-10 [J. Pharm. Biomed. Anal. 116 , 101-4, (2015)] |
|
Separation and quantitation of the enantiomers of methamphet...
1995-01-01 [J. Anal. Toxicol. 19(3) , 139-47, (1995)] |
|
Indirect resolution of beta-blocker agents by reversed-phase...
2004-02-01 [Electrophoresis 25(4-5) , 607-14, (2004)] |
|
Recent progress in liquid chromatographic enantioseparation ...
1996-01-01 [Biomed. Chromatogr. 10(6) , 265-77, (1996)] |
|
Chiral separation and detection enhancement of propranolol u...
1993-02-01 [J. Pharm. Biomed. Anal. 11(2) , 117-20, (1993)] |