Chiral separation and detection enhancement of propranolol using automated pre-column derivatization.
F Lai, A Mayer, T Sheehan
Index: J. Pharm. Biomed. Anal. 11(2) , 117-20, (1993)
Full Text: HTML
Abstract
In the liquid chromatographic analysis of pharmaceuticals, two challenges are often encountered: detectivity and chiral separations. Propranolol, a beta adrenergic blocker, is a pharmaceutical compound that faces both of these limitations. In this study, both limitations are overcome simultaneously using derivatization with (+)-1-(9-fluorenyl)ethyl chloroformate (FLEC), a highly fluorescent and chiral reagent. The derivatization is automated using an autosampler with an AutoMix microrobotic feature, which greatly contributes to the efficiency and reproducibility of the method when manipulating microliter volumes of sample and reagents. The method yields excellent separation of the diasteriomers, has a detection limit of 1 picomol, good reproducibility and linearity in the 50-400 pmol range (on column). In addition, this method is simple, requires no elevated temperature, no chiral stationary or mobile phases and can be easily automated.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
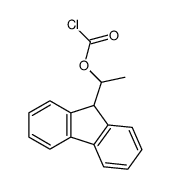 |
(+)-1-(9-fluorenyl)ethyl chloroformate
CAS:107474-79-3 |
C16H13ClO2 |
|
Enantioselective analysis of D- and L-amino acids from mouse...
2015-12-10 [J. Pharm. Biomed. Anal. 116 , 101-4, (2015)] |
|
Separation and quantitation of the enantiomers of methamphet...
1995-01-01 [J. Anal. Toxicol. 19(3) , 139-47, (1995)] |
|
Indirect resolution of beta-blocker agents by reversed-phase...
2004-02-01 [Electrophoresis 25(4-5) , 607-14, (2004)] |
|
Recent progress in liquid chromatographic enantioseparation ...
1996-01-01 [Biomed. Chromatogr. 10(6) , 265-77, (1996)] |
|
(+)-Fluorenylethylchloroformate (FLEC)--improved synthesis f...
2013-04-28 [Org. Biomol. Chem. 11(16) , 2571-3, (2013)] |