| Structure | Name/CAS No. | Articles |
|---|---|---|
 |
Chloramphenicol
CAS:56-75-7 |
|
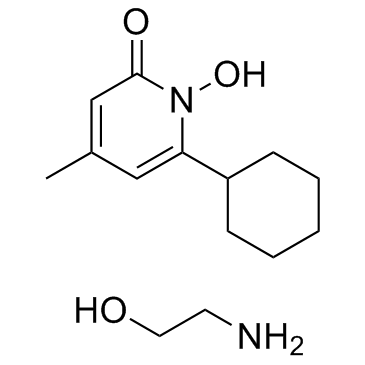 |
Ciclopirox Olamine
CAS:41621-49-2 |
|
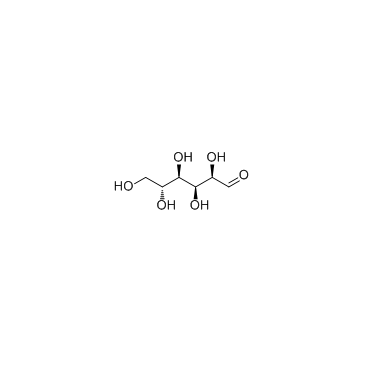 |
D-(+)-Glucose
CAS:50-99-7 |
|
 |
magnesium sulfate
CAS:7487-88-9 |
|
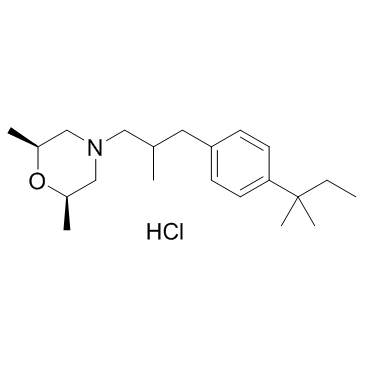 |
Amorolfine hydrochloride
CAS:78613-38-4 |
|
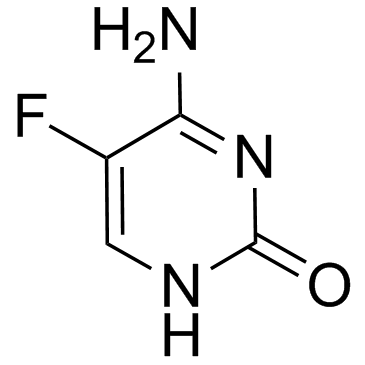 |
5-Flucytosine
CAS:2022-85-7 |
|
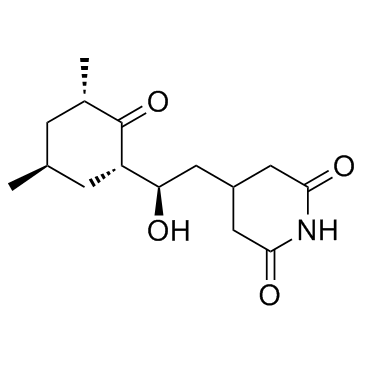 |
Cycloheximide
CAS:66-81-9 |
|
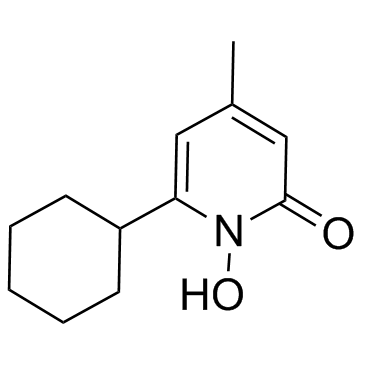 |
Ciclopirox
CAS:29342-05-0 |