| Structure | Name/CAS No. | Articles |
|---|---|---|
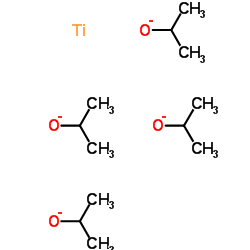 |
Titanium(4+) tetrapropan-2-olate
CAS:546-68-9 |
|
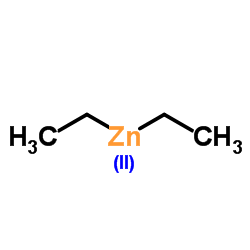 |
Diethylzinc
CAS:557-20-0 |